OptiPrepTM多功能分离液是质量浓度为60%(w/v)的碘克砂醇溶液,密度为1.32 g/mL。
OptiPrepTM Reference List RC06“从大脑和脊髓中分离神经细胞”中提供了一个所有使用OptiPrepTM多功能分离液已发表论文的全面参考书目:https://diagnostic.serumwerk.com/references-2/cell-references-serumwerk/。
如需获取文本中提到的其他应用手册,http://www.shanjin.com.cn/news/3.html,页面按数字即可进行查询。
01 背景
在神经组织中,有许多类型的“支持细胞”,它们被归类为“神经胶质细胞”,本应用说明书只涉及其中的两种。在应用抗体结合微球纯化少突胶质细胞之前,碘克砂醇梯度法就已被用于纯化神经胶质细胞并且从分离的中枢神经系统组织中去除髓磷脂。
小胶质细胞已经通过组织剪碎后,用papain[1]或trypsin[2]消化细胞的标准方法从小鼠大脑中分离出来了。它们也已经从体外分化至第6个阶段的小鼠胚胎干细胞的培养物中被分离成功[2]。后者包括未分化干细胞的扩增,随后是胚状体的产生,巢蛋白阳性细胞的选择和扩增,分化为神经元和小胶质细胞的扩增。该方法的操作细节超出了本节应用手册的范围,详细信息请参阅参考文献[2]。
02 小鼠脑胶质细胞的纯化
对于梯度纯化,Bettinger等[1]采用了四步梯度法,梯度溶液上层覆盖样品溶液,而Tsuchiya等[2]采用了两步梯度浮选的策略,这两个方法都有在Protocol中列出。最近O 'Mahony等[3]也使用了四步梯度法来分离少突胶质细胞、神经元+胶质副细胞和小胶质细胞。
2a. 试剂
A. OptiPrepTM分离液(使用前轻轻摇晃瓶身)
B. Hank's平衡盐溶液(Hank’s Balanced Salt Solution,HBSS),详见Note 1
2b. Protocol
1. 用Solution B对OptiPrepTM进行稀释,制备成不同浓度碘克砂醇溶液:4%、5.5%、7%和10%(w/v)的碘克砂醇溶液(四步梯度法)[1],或者9.6%和21.6%(w/v)的碘克砂醇溶液(两步梯度法)[2],详见Note 2。
2. 剪碎脑组织,然后用70 μm的筛网进行过滤。
3. 用Solution B对剪碎的组织进行重悬,选用下面的方法之一对组织进行分离:
a. 每个脑组织用500 μg papain室温消化20 min,再用1 mg/mL DNase I室温消化5 min[1];
b. 0.25% trypsin和0.1 mg/mL DNase I室温孵育20 min[2];
4. 用Solution B稀释细胞悬液,1500×g离心,10 min,弃上清,详见 Note 3。
5. 用Solution B重悬细胞沉淀(四步梯度法),或者用21.6%(w/v)的碘克砂醇溶液重悬细胞沉淀(两步梯度法)。
6. 分别取4%、5.5%、7%和10%(w/v)的碘克砂醇溶液各1 mL,按照顺序平铺在离心管内,最后将细胞悬液平铺在碘克砂醇溶液的上层(四步梯度法),或者取等体积的9.6%(w/v)碘克砂醇溶液平铺在细胞悬液的上层(两步梯度法),详见Notes 4和5。
7. 3000×g离心,20 min(四步梯度法),或者670×g离心,20 min(两步梯度法),详见Notes 6和7。
8. 四步梯度法分离得到的小胶质细胞带位于最下层。两步梯度法分离得到的小胶质细胞带位于中间层,详见Notes 7-9。
2c. Notes
1. 适配的溶液——缓冲盐溶液或培养基都可以用来代替Hank's平衡盐溶液。
2. O'Mahony等[3]采用Hibernate A/B27培养基对OptiPrepTM稀释,设置梯度分别为9%、12%、15%和21%(w/v)的碘克砂醇溶液。而近期Song等[4]和Hong等[5]则采用7.5%、10.0%、13.5%和17% (w/v)的碘克砂醇溶液,800×g离心,15 min,弃上清,分离得到小胶质细胞。
3. Tsuchiya等[2]在这一步操作中的离心时间为5 min。
4. 较大的梯度体积可以提高分离效果[3]。
5. 不连续的梯度通常最容易通过使用2 mL注射器和长金属套管进行分层制备(即先加入低密度溶液)。对于叠层的溶液制备是非常困难的,尤其是那些密度相差很小的溶液。关于梯度溶液制备的更多信息详见Application Sheet C02。
6. Tsuchiya等[2]在这一步设定温度是24℃。
7. O’Mahony等[3]的梯度系统设置为800×g离心,15 min。离心后,丢弃梯度最上层的6 mL含碎片溶液,在高浓度梯度带溶液中可观察到的细胞包含:
(1)寡树突胶质细胞;
(2)神经元+胶质副细胞;
(3)神经细胞;
(4)小神经胶质细胞。
8. 密度为6%或6.2%(w/v)的碘克砂醇分离系统也已经被用于分离神经元和神经胶质细胞[6-10]。
9. Tucsek等[11]采用了屏障浮选法,在密度为21%(w/v)的碘克砂醇溶液上层平铺密度为9.6%的碘克砂醇溶液,670×g离心,20 min,从分界面处可获得小胶质细胞。
03 去除髓磷脂纯化大鼠脑细胞组分
为从大鼠的中脑和小脑组织中分离少突胶质细胞,需要配制合适的细胞悬浮液,而过程中所涉及的培养和孵育过程是非常复杂的,并且可能因不同实验室而有所差异。有关这些方法的详细信息可以查询Section 4。下面这个简短的说明只涉及简单的一步梯度方法去除髓磷脂:将分离的细胞悬液与OptiPrepTM混合,使碘克砂醇终浓度为9%(w/v),800×g离心,20 min。丢弃上清中的髓磷脂,将分离得到的细胞重悬于合适的培养基内[12-16]。
04 其他神经元细胞纯化
少突胶质细胞是通过OptiPrepTM分离液采用四步梯度法来制备分离的,纯度>90%[3,17-19]。梯度类似于Application Sheet C36索引“脑运动神经元的分离”一文中的描述。少突胶质细胞通常分布在密度相对较低的上层。
星形胶质细胞也是通过四步梯度法的碘克砂醇溶液纯化得到[20]。首先用10 mM MOPS-137 mM NaCl稀释OptiPrepTM,得到密度约29.7%(w/v)的碘克砂醇工作溶液(WS,ρ=1.161 g/mL)。进一步稀释碘克砂醇工作液需要使用完全培养基(含有10% FBS的DMEM,密度约为1.009 g/mL)得到表1所示的四种溶液。
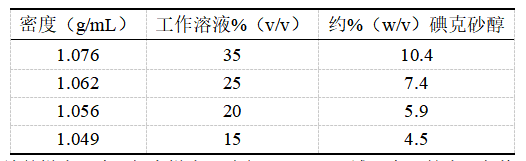
对于不连续的梯度分离,每个梯度溶液各取1 mL平铺于离心管内,在其上方覆盖6 mL粗制的细胞悬液,约800×g离心,15 min[20]。星形胶质细胞呈带状分布于密度1.062 g/mL和密度1.056 g/mL的碘克砂醇溶液分界处。这些细胞也使用OptiPrepTM Application Sheet C36中描述的四步碘克砂醇梯度法进行纯化[21],详见上文。
碘克砂醇梯度法对研究创伤性脊髓损伤后的炎症反应也非常有价值。通过将分离的脊髓细胞铺在4.5%、6%、7.5%和10.5%(w/v)碘克砂醇溶液上(OptiPrepTM先用0.15 M NaCl, 10 mM MOPS,pH 7.4稀释成30%的碘克砂醇溶液,然后用Hank’s平衡盐溶液进一步稀释),1900 rpm离心,15 min。最上层是残留的碎片,神经细胞分布在三个较低溶液界面处呈带状,炎症细胞和神经胶质细胞沉淀在试管底部[22,23]。Beck[24]等将OptiPrepTM与PercollTM以及其他方法进行比较,发现只有OptiPrepTM梯度分离法能够准确定量评估脊髓损伤组织中PMNs的存在。
参考文献[25]给出了一些基于OptiPrepTM技术的方法学论述。
05 参考文献
[1] Bettinger, I., Thanos, S., Paulus, W., 2002. Microglia promote glioma migration. Acta Neuropathol 103(4), 351-355.
[2] Tsuchiya, T., Park, K.C., Toyonaga, S., Yamada, S.M., Nakabayashi, H., Nakai, E., Ikawa, N., Furuya, M., Tominaga, A., Shimizu, K., 2005. Characterization of microglia induced from mouse embryonic stem cells and their migration into the brain parenchyma. J Neuroimmunol 160(1-2), 210-218.
[3] O'Mahony, A., Raber, J., Montano, M., Foehr, E., Han, V., Lu, S.M., Kwon, H., LeFevour, A., Chakraborty-Sett, S., Greene, W.C., 2006. NF-kappaB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol 26(19), 7283-7298.
[4] Song, D.Y., Yu, H.N., Park, C.R., Lee, J.S., Lee, J.Y., Park, B.G., Woo, R.S., Han, J.T., Cho, B.P., Baik, T.K., 2013. Down-regulation of microglial activity attenuates axotomized nigral dopaminergic neuronal cell loss. BMC Neurosci 14, 112.
[5] Hong, H., Krause, H.J., Sohn, S., Baik, T., Park, J.H., Shin, S., Park, C., Song, D., 2014. In situ measurement of superoxide and hydroxyl radicals by frequency mixing detection technique. Anal Biochem 447, 141-145.
[6] Goethals, S., Ydens, E., Timmerman, V., Janssens, S., 2010. Toll-like receptor expression in the peripheral nerve. Glia 58(14), 1701-1709.
[7] Papa, S., Rossi, F., Ferrari, R., Mariani, A., De Paola, M., Caron, I., Fiordaliso, F., Bisighini, C., Sammali, E., Colombo, C., Gobbi, M., Canovi, M., Lucchetti, J., Peviani, M., Morbidelli, M., Forloni, G., Perale, G., Moscatelli, D., Veglianese, P., 2013. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano 7(11), 9881-9895.
[8] Papa, S., Ferrari, R., De Paola, M., Rossi, F., Mariani, A., Caron, I., Sammali, E., Peviani, M., Dell'Oro, V., Colombo, C., Morbidelli, M., Forloni, G., Perale, G., Moscatelli, D., Veglianese, P., 2014. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J Control Release 174, 15-26.
[9] Mariani, A., Fanelli, R., Re Depaolini, A., De Paola, M., 2015. Decabrominated diphenyl ether and methylmercury impair fetal nervous system development in mice at documented human exposure levels. Dev Neurobiol 75(1), 23-38.
[10] Papa, S., Caron, I., Erba, E., Panini, N., De Paola, M., Mariani, A., Colombo, C., Ferrari, R., Pozzer, D., Zanier, E.R., Pischiutta, F., Lucchetti, J., Bassi, A., Valentini, G., Simonutti, G., Rossi, F., Moscatelli, D., Forloni, G., Veglianese, P., 2016. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials 75, 13-24.
[11] Tucsek, Z., Toth, P., Sosnowska, D., Gautam, T., Mitschelen, M., Koller, A., Szalai, G., Sonntag, W.E., Ungvari, Z., Csiszar, A., 2014. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. J Gerontol A Biol Sci Med Sci 69(10), 1212-1226.
[12] Chari, D.M., Crang, A.J., Blakemore, W.F., 2003. Decline in rate of colonization of oligodendrocyte progenitor cell (OPC)-depleted tissue by adult OPCs with age. J Neuropathol Exp Neurol 62(9), 908-916.
[13] Li, G., Crang, A.J., Rundle, J.L., Blakemore, W.F., 2002. Oligodendrocyte progenitor cells in the adult rat CNS express myelin oligodendrocyte glycoprotein (MOG). Brain Pathol 12(4), 463-471.
[14] Luyt, K., Varadi, A., Halfpenny, C.A., Scolding, N.J., Molnar, E., 2004. Metabotropic glutamate receptors are expressed in adult human glial progenitor cells. Biochem Biophys Res Commun 319(1), 120-129.
[15] Crang, A.J., Gilson, J.M., Li, W.W., Blakemore, W.F., 2004. The remyelinating potential and in vitro differentiation of MOG-expressing oligodendrocyte precursors isolated from the adult rat CNS. Eur J Neurosci 20(6), 1445-1460.
[16] Janes, K., Wahlman, C., Little, J.W., Doyle, T., Tosh, D.K., Jacobson, K.A., Salvemini, D., 2015. Spinal neuroimmune activation is independent of T-cell infiltration and attenuated by A3 adenosine receptor agonists in a model of oxaliplatin-induced peripheral neuropathy. Brain Behav Immun 44, 91-99.
[17] Donati, D., Akhyani, N., Fogdell-Hahn, A., Cermelli, C., Cassiani-Ingoni, R., Vortmeyer, A., Heiss, J.D., Cogen, P., Gaillard, W.D., Sato, S., Theodore, W.H., Jacobson, S., 2003. Detection of human herpesvirus-6 in mesial temporal lobe epilepsy surgical brain resections. Neurology 61(10), 1405-1411.
[18] Cassiani-Ingoni, R., Greenstone, H.L., Donati, D., Fogdell-Hahn, A., Martinelli, E., Refai, D., Martin, R., Berger, E.A., Jacobson, S., 2005. CD46 on glial cells can function as a receptor for viral glycoprotein-mediated cell-cell fusion. Glia 52(3), 252-258.
[19] Sotnikov, I., Veremeyko, T., Starossom, S.C., Barteneva, N., Weiner, H.L., Ponomarev, E.D., 2013. Platelets recognize brain-specific glycolipid structures, respond to neurovascular damage and promote neuroinflammation. PLoS One 8(3), e58979.
[20] Kerstetter, A.E., Miller, R.H., 2012. Isolation and culture of spinal cord astrocytes. Methods Mol Biol 814, 93-104.
[21] Freeman, K.A., Fullerton, D.A., Foley, L.S., Bell, M.T., Cleveland, J.C., Jr., Weyant, M.J., Mares, J., Meng, X., Puskas, F., Reece, T.B., 2015. Spinal cord protection via alpha-2 agonist-mediated increase in glial cell-line-derived neurotrophic factor. J Thorac Cardiovasc Surg 149(2), 578-584; discussion 584-576.
[22] Galvan, M.D., Luchetti, S., Burgos, A.M., Nguyen, H.X., Hooshmand, M.J., Hamers, F.P., Anderson, A.J., 2008. Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. J Neurosci 28(51), 13876-13888.
[23] Nguyen, H.X., Galvan, M.D., Anderson, A.J., 2008. Characterization of early and terminal complement proteins associated with polymorphonuclear leukocytes in vitro and in vivo after spinal cord injury. J Neuroinflammation 5, 26.
[24] Beck, K.D., Nguyen, H.X., Galvan, M.D., Salazar, D.L., Woodruff, T.M., Anderson, A.J., 2010. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133(Pt 2), 433-447.
[25] Vincent, A.M. and Feldman, E.L. (2010) Primary sensory and motor neuron cultures In, Protocols for Neural Cell Culture, Springer Protocols Handbooks, (ed. Doering, L.C.), Humana Press (Springer Science+Business Media), Totowa, NJ. pp 161-173.
OptiPrepTM Application Sheet C35;第八版,2020年2月
转自(有修改):https://mp.weixin.qq.com/s/UU5O5joYFYTOxjpk_23xkA

 手机/微信
手机/微信